Introducing the atomic structure webquest answer key, an indispensable guide that empowers you to delve into the intricate realm of atomic structure. This comprehensive resource provides a structured approach to understanding the fundamental components of matter, unlocking the secrets that govern the behavior of elements and shaping our world.
Delving into the key concepts of atomic structure, we explore the nature of atoms, their subatomic constituents, and the arrangement of these particles within an atom. The electron configuration, a defining characteristic of elements, is thoroughly examined, highlighting its impact on chemical properties and the organization of elements in the periodic table.
Atomic Structure: Atomic Structure Webquest Answer Key

Atomic structure refers to the internal composition and arrangement of matter at the atomic level. Understanding atomic structure is crucial as it forms the basis of chemistry, materials science, and other scientific disciplines. A webquest is an interactive learning tool that guides students through exploring and understanding atomic structure in a structured and engaging manner.
Key Concepts, Atomic structure webquest answer key
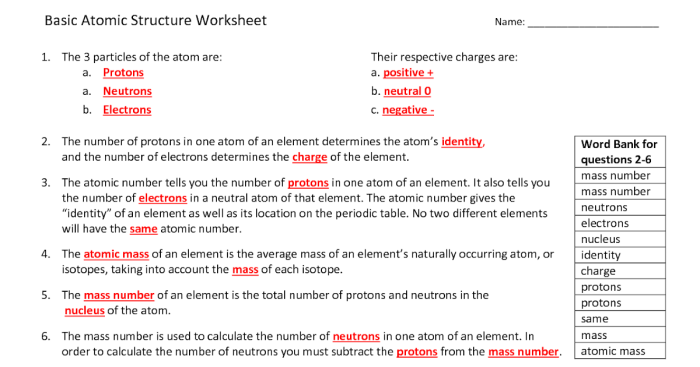
Atoms are the fundamental building blocks of matter, composed of a nucleus and surrounding electrons. The nucleus contains positively charged protons and neutral neutrons, while electrons are negatively charged and orbit the nucleus in specific energy levels called electron shells.
Electron Configuration
Electron configuration describes the distribution of electrons within an atom’s electron shells. The periodic table organizes elements based on their electron configurations, which determine their chemical properties and behavior.
Atomic Orbitals
Atomic orbitals are mathematical functions that describe the probability of finding an electron in a specific region of space around the nucleus. Different types of orbitals (s, p, d, f) have distinct shapes and energy levels, influencing the bonding behavior of atoms.
Applications of Atomic Structure
Understanding atomic structure has practical applications in various fields:
- Materials science: Designing new materials with enhanced properties
- Chemistry: Predicting chemical reactions and developing new molecules
- Medicine: Developing drugs and treatments based on atomic-level interactions
- Nuclear physics: Understanding nuclear reactions and energy production
- Astrophysics: Studying the composition and behavior of celestial objects
Popular Questions
What is the purpose of an atomic structure webquest?
An atomic structure webquest is designed to provide a structured and interactive learning experience, guiding students through the exploration of atomic structure concepts through online resources.
What are the key subatomic particles that make up atoms?
Atoms are composed of protons, neutrons, and electrons, each with distinct properties and roles within the atom.
How does electron configuration affect the chemical behavior of elements?
Electron configuration determines the number of valence electrons in an atom, which in turn influences its chemical reactivity and bonding behavior.