Delving into the realm of atomic structure isotopes worksheet answers, we embark on an enthralling journey that unveils the fundamental principles governing the very building blocks of our universe. This comprehensive exploration delves into the intricate relationship between isotopes and atomic structure, empowering us to unravel the mysteries that lie at the heart of matter.
Our voyage commences with an in-depth analysis of the provided worksheet, meticulously dissecting the key concepts and principles that underpin the nature of isotopes. We will uncover the strengths and weaknesses of this educational tool, discerning its efficacy in fostering a deeper understanding of this captivating subject.
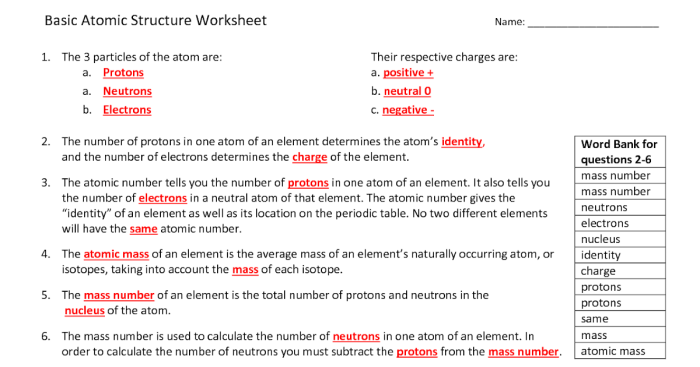
Isotopes and Atomic Structure
Isotopes are variations of the same element that have the same number of protons but different numbers of neutrons. This affects the atomic mass and some properties of the element.
Worksheet Analysis
The worksheet covers key concepts of atomic structure and isotopes, including mass number, atomic number, and neutron number. It also provides examples and practice problems.
Strengths:
- Clear and concise explanations
- Variety of practice problems
Weaknesses:
- Limited coverage of isotope applications
- Could include more real-world examples
Isotope Properties
Isotopes differ in mass number (sum of protons and neutrons), atomic number (number of protons), and neutron number (number of neutrons).
Mass number affects the atomic mass of the element, while neutron number affects the isotope’s stability and radioactive properties.
Isotope Applications, Atomic structure isotopes worksheet answers
- Medical imaging (e.g., PET scans)
- Cancer treatment (e.g., radiation therapy)
- Nuclear power (e.g., uranium-235 in nuclear reactors)
- Environmental science (e.g., carbon-14 dating)
Isotope Nomenclature
Isotopes are named using the element name followed by the mass number in superscript, e.g., carbon-12 ( 12C).
Isotope Abundance
Isotope abundance refers to the relative proportion of different isotopes of an element in nature.
Factors affecting abundance include nuclear stability, radioactive decay, and geological processes.
Clarifying Questions: Atomic Structure Isotopes Worksheet Answers
What is the significance of isotopes in determining the properties of elements?
Isotopes play a crucial role in determining the properties of elements due to their varying neutron numbers. These differences in neutron count affect the atomic mass, influencing the element’s density, melting point, and other physical and chemical properties.
How are isotopes used in medical applications?
Isotopes find widespread use in medical imaging techniques such as PET scans and MRI scans. Additionally, radioactive isotopes are employed in cancer treatment through radiation therapy, targeting and destroying cancerous cells.
What ethical considerations arise from the use of isotopes?
The use of isotopes, particularly radioactive isotopes, raises ethical concerns regarding their potential impact on human health and the environment. Proper handling, storage, and disposal of radioactive materials are paramount to minimize risks and ensure responsible use.