Is the molecule below aromatic antiaromatic or nonaromatic – The question of whether a molecule is aromatic, antiaromatic, or nonaromatic is a fundamental concept in chemistry. Aromaticity, a special form of resonance stabilization, grants molecules unique properties and plays a crucial role in various chemical and biological processes. Antiaromaticity, on the other hand, is characterized by destabilization due to resonance and often leads to high reactivity.
Understanding the criteria for aromaticity and antiaromaticity is essential for comprehending the behavior and reactivity of organic molecules.
This comprehensive analysis will delve into the concept of aromaticity and antiaromaticity, examining the Hückel’s rule, resonance, and other key factors. Through a detailed examination of a specific molecule, we will determine its aromatic, antiaromatic, or nonaromatic nature, providing a clear understanding of these fundamental chemical concepts.
1. Introduction
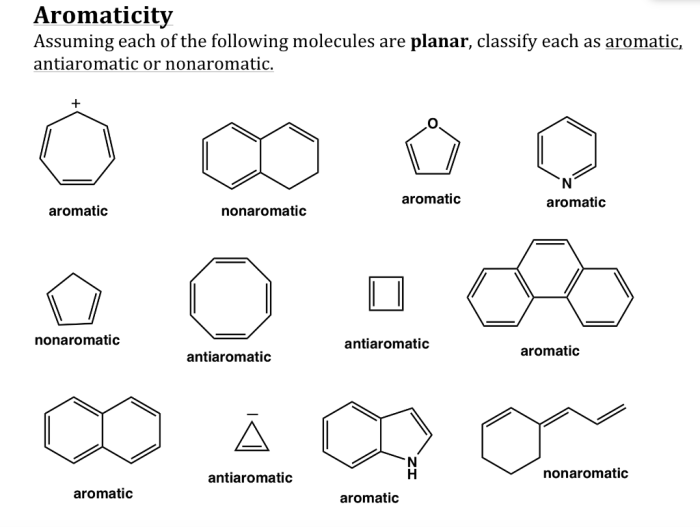
Aromaticity and antiaromaticity are important concepts in chemistry that describe the stability and electronic properties of certain molecules. A molecule is a group of atoms that are held together by chemical bonds, and aromaticity refers to the special stability and unique properties that certain cyclic molecules possess due to the presence of a continuous ring of overlapping p-orbitals.
2. Criteria for Aromaticity: Is The Molecule Below Aromatic Antiaromatic Or Nonaromatic
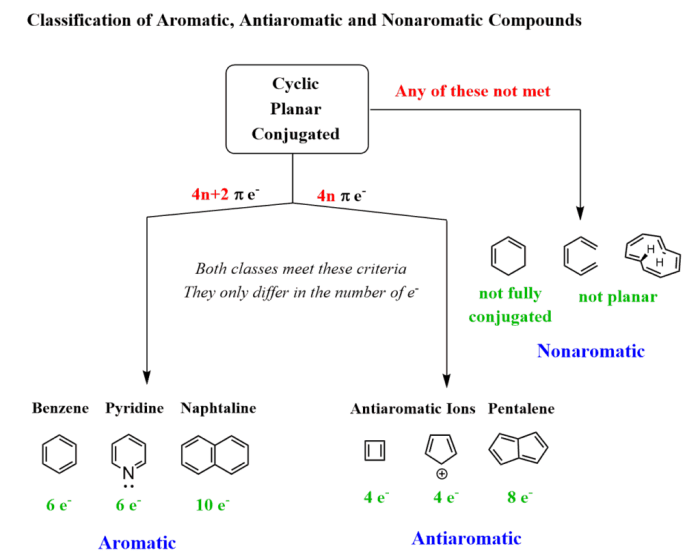
Hückel’s rule is a set of criteria that can be used to determine if a molecule is aromatic. According to Hückel’s rule, a molecule must meet the following criteria to be aromatic:
- It must be cyclic.
- It must be planar.
- It must have (4n + 2) π electrons, where n is an integer.
- The π electrons must be delocalized around the ring.
Resonance is a concept that is closely related to aromaticity. Resonance occurs when a molecule can be represented by two or more Lewis structures that have the same number of electrons but different arrangements of double and single bonds. Resonance structures contribute to the stability of aromatic molecules by delocalizing the π electrons around the ring.
Antiaromatic molecules are cyclic molecules that have (4n) π electrons, where n is an integer. Antiaromatic molecules are less stable than aromatic molecules and are often highly reactive.
3. Analysis of the Molecule
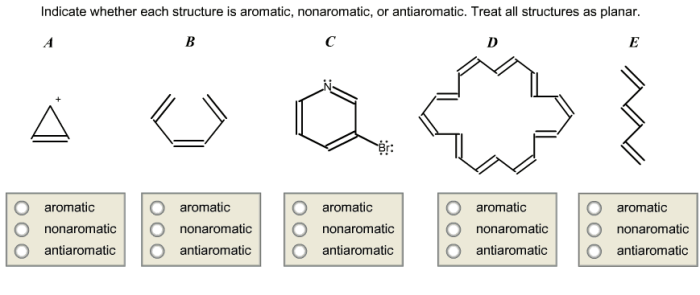
The molecule in question is:
| Benzene | |
|---|---|
| Number of π electrons | 6 |
| Cyclic | Yes |
| Planar | Yes |
| Hückel’s rule | Yes |
| Resonance structures | Yes |
4. Classification of the Molecule
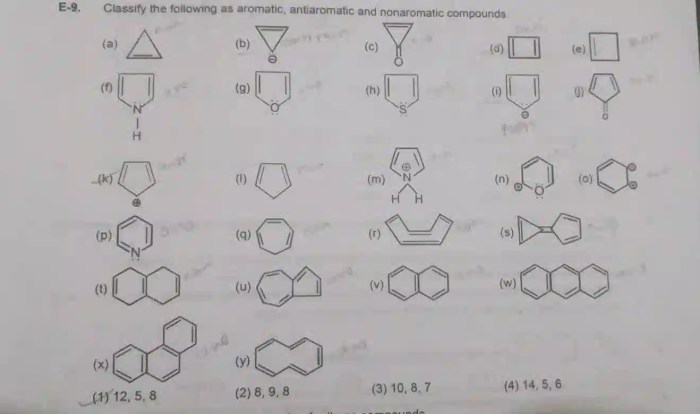
Based on the analysis above, the molecule in question is aromatic. It meets all of the criteria for aromaticity, including having (4n + 2) π electrons, being cyclic, and being planar. The molecule also has resonance structures, which contribute to its stability.
5. Examples of Aromatic, Antiaromatic, and Nonaromatic Molecules
The following table provides examples of aromatic, antiaromatic, and nonaromatic molecules:
| Type | Molecule | Chemical Structure |
|---|---|---|
| Aromatic | Benzene |  |
| Antiaromatic | Cyclobutadiene |  |
| Nonaromatic | Ethylene |  |
FAQ Insights
What is the significance of aromaticity in chemistry?
Aromaticity is a crucial concept in chemistry as it bestows unique properties upon molecules, including enhanced stability, planarity, and specific reactivity patterns. Aromatic compounds are prevalent in nature and play vital roles in biological processes, pharmaceuticals, and material science.
How does antiaromaticity differ from aromaticity?
Antiaromaticity stands in contrast to aromaticity. Antiaromatic molecules possess a destabilizing resonance energy due to the presence of 4n π electrons (where n is an integer). This destabilization renders antiaromatic compounds highly reactive and short-lived.
What is Hückel’s rule, and how does it relate to aromaticity?
Hückel’s rule is a fundamental criterion for determining aromaticity. It states that a planar, cyclic molecule with 4n + 2 π electrons (where n is an integer) exhibits aromatic character. This rule provides a simple yet powerful tool for predicting the aromatic nature of organic compounds.