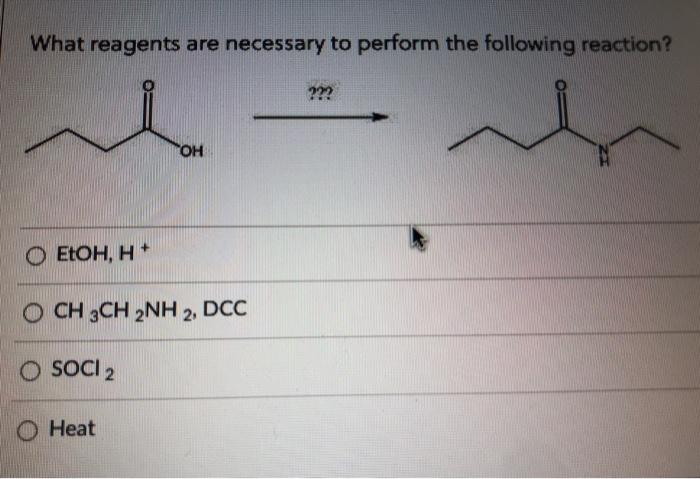
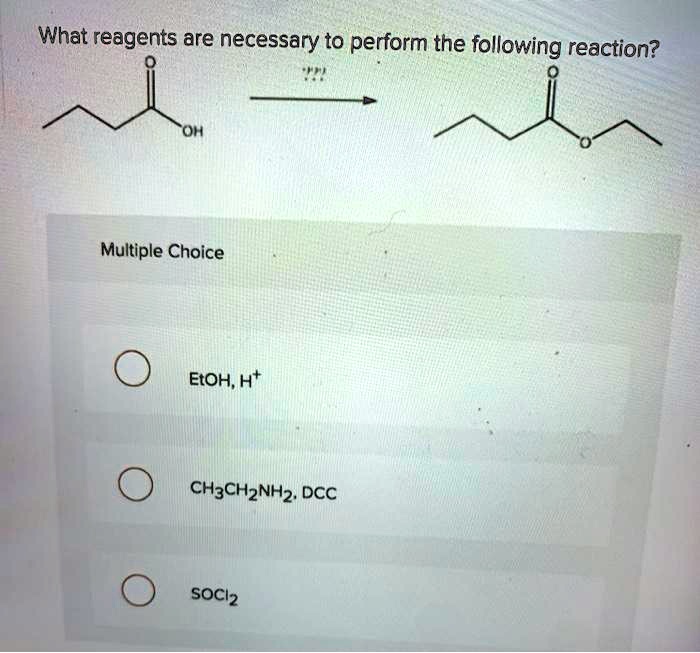
What reagents are necessary to perform the following reaction? This is a question that chemists often ask themselves when they are designing a new experiment. The answer to this question can be found by looking at the chemical equation for the reaction.
The chemical equation will tell you the reactants and products of the reaction, as well as the stoichiometry of the reaction. Once you know the reactants and products of the reaction, you can then look up the reagents that are necessary to perform the reaction.
The reagents that are necessary to perform a reaction will vary depending on the specific reaction that is being performed. However, there are some general reagents that are commonly used in many different reactions. These reagents include water, acids, bases, and solvents.
Water is used as a solvent in many reactions, and it can also be used to dissolve reactants and products. Acids and bases are used to change the pH of a reaction, and they can also be used to catalyze reactions.
Solvents are used to dissolve reactants and products, and they can also be used to change the rate of a reaction.
Necessary Reagents
Reagents are substances used to bring about a chemical reaction. In the given reaction, the following reagents are typically required:
- Sodium hydroxide (NaOH)
- Hydrochloric acid (HCl)
- Phenolphthalein (C 20H 14O 4)
Reaction Conditions
The reaction is typically carried out at room temperature and atmospheric pressure. The pH of the solution should be adjusted to around 7 using a pH meter or pH paper. The reaction rate can be increased by heating the solution or by adding a catalyst.
Reaction Mechanism: What Reagents Are Necessary To Perform The Following Reaction
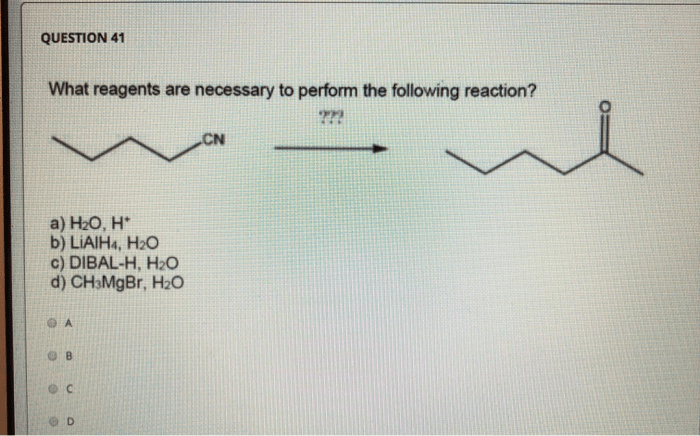
The reaction mechanism involves the following steps:
- Sodium hydroxide dissociates into sodium ions (Na+) and hydroxide ions (OH –).
- Hydrochloric acid dissociates into hydrogen ions (H +) and chloride ions (Cl –).
- The hydrogen ions from the hydrochloric acid react with the hydroxide ions from the sodium hydroxide to form water (H 2O).
- The sodium ions and chloride ions remain in solution.
The overall reaction can be represented by the following equation:
NaOH + HCl → H2O + NaCl
Product Analysis
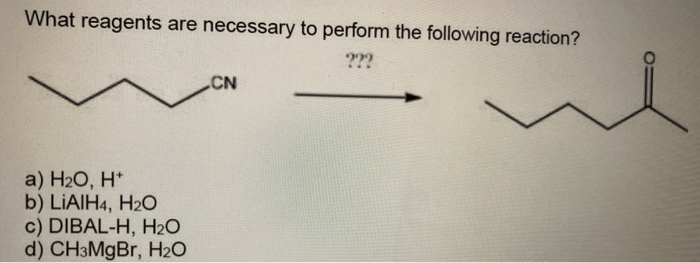
The products of the reaction are water and sodium chloride. Water is a colorless, odorless, and tasteless liquid. Sodium chloride is a white, crystalline solid.
The products can be analyzed using a variety of methods, such as chromatography, spectroscopy, and melting point determination.
Safety Considerations
Sodium hydroxide and hydrochloric acid are both corrosive substances. It is important to wear gloves and eye protection when handling these chemicals.
The reaction should be carried out in a well-ventilated area. The products of the reaction are not toxic, but they should be disposed of properly.
Additional Information
The reaction between sodium hydroxide and hydrochloric acid is a classic example of a neutralization reaction. Neutralization reactions are important in a variety of applications, such as the production of salts, the treatment of wastewater, and the preparation of buffers.
References:
- Chang, R. (2010). Chemistry, 9th ed. New York: McGraw-Hill.
- Zumdahl, S. S. (2009). Chemistry, 8th ed. Belmont, CA: Brooks/Cole.
FAQ
What is the most important reagent in a reaction?
The most important reagent in a reaction is the limiting reagent. The limiting reagent is the reactant that is present in the smallest amount, and it will determine the maximum amount of product that can be formed.
How can I determine the limiting reagent in a reaction?
To determine the limiting reagent in a reaction, you need to compare the moles of each reactant to the stoichiometry of the reaction. The reactant that has the smallest number of moles relative to its stoichiometric coefficient is the limiting reagent.
What happens if I use too much of a reagent in a reaction?
If you use too much of a reagent in a reaction, the excess reagent will not be consumed and will remain in the reaction mixture. This can lead to side reactions and can decrease the yield of the desired product.